CREATING CONFIDENCE
MANUFACTURING
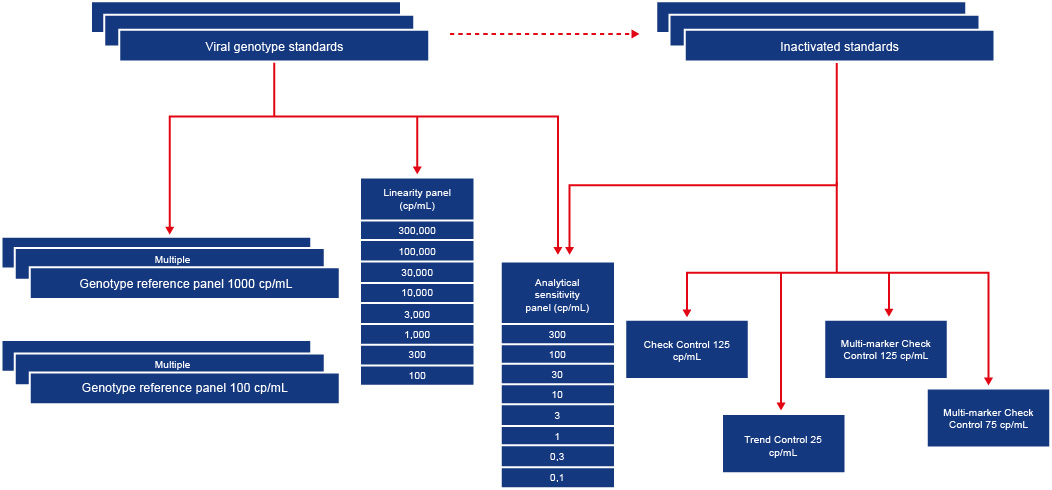
Since organising the first international proficiency study of viral NAT methods in the early 1990s (Lancet 1993:441:722-724), our deep frozen viral plasma standards in liquid nitrogen have shown to be stable at -80˚C. A fully traceable manufacturing system of gravimetrically recorded dilutions allows for reproducible production of batches of reference samples of known viral concentration. The batch release control process ensures consistent reactivity of product comparable to the first manufactured reference batch. Our reference standards for viral serologic assays and NAT methods are diluted in the same serum or plasma matrix as clinical samples. The viral safety of run controls is guaranteed by inactivation methods of proven efficacy in the plasma and vaccine industry. Our ISO 13485 certified manufacturing facility is secured for long term storage of standard dilutions at -80˚C or -30˚C (depending on the stability of the analyte). At regular intervals the facility is audited by the Notified Body (MDC) for ISO13485:2016. The figure below gives an overview of the different types of reference panels and run controls that are manufactured for NAT assays. A series of run controls for viral serology and NAT have been CE marked by the Notified Body (MDC), while others are under review.
ABOUT
- Mission
- Manufacturing
- History
- Management Team