CREATING CONFIDENCE
MISSION
Our mission is to provide consistent reference standards for validation and quality control of assays for detection of blood borne pathogens.
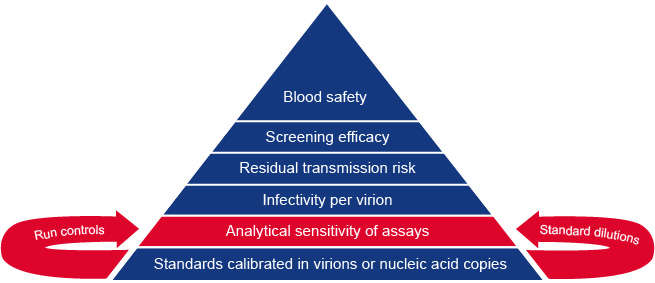
Our ambition is to provide a post-market performance follow up system for in vitro diagnostic devices (IVDs) intended for viral safety testing of blood products. This can be achieved by external quality control ensuring sufficient analytical sensitivity of blood screening tests. In understanding residual viral transmission risk by blood transfusion it is important that standards for validating NAT assays are calibrated in nucleic acid copies or virion numbers. The figure explains the foundations of our system for monitoring blood safety testing.
ABOUT
- Mission
- Manufacturing
- History
- Management Team
Figure. Standards for reference panels and run controls: foundations for monitoring blood safety